Genetic 'scissors' conquer the body
The discovery of CRISPR dates back to the early 1990s, when Spanish microbiologist Francisco Mojica discovered a series of repetitive sequences in the DNA of extremophilic bacteria, in particular bacteria adapted to live in high-salinity environments. Normally, DNA is a diverse coding sequence, a book of instructions that cells need to make proteins. The repeats observed by Mojica were an anomaly. They were also curious in that they were almost palindromic: they could be read backwards and forwards. In 1993, he published a paper in which he identified these repetitive sequences and called them "clustered regularly interspaced short palindromic repeats", also abbreviated as CRISPR. Although this discovery initially went unnoticed, a decade later the scientific community became interested in the origin and evolutionary reasons behind these mysterious palindromic sequences. In 2007, scientists discovered that CRISPR sequences were part of the immune system of bacteria, which used this genetic technology to 'learn' how to fight viruses and resist infection.
Soon after, the real revolution came. In the early 2010s, scientists began to exploit the same CRISPR system that bacteria use to protect themselves as a genome editing tool. In 2012, researchers Jennifer Doudna and Emmanuelle Charpentier, winners of the Nobel Prize in Chemistry a few years later, published a groundbreaking paper describing how they could reprogram the CRISPR system to "cut and paste" specific DNA sequences in the lab. Doudna and Charpentier demonstrated that they could design an RNA guide to direct an enzyme, called Cas9, to specific DNA sequences, then cut them with unprecedented precision. This process allowed scientists to edit the genome with unique ease and precision. Since then, CRISPR technology has undergone rapid development, to the point where there are now effective treatments for humans with genetic diseases such as sickle cell anaemia, developed by the pharmaceutical company Vertex and authorised by UK and US regulatory agencies. In addition, several variants of the original technique have been developed, with more advanced enzymes such as CRISPR-Cas12 and CRISPR-Cas13, which allow genome editing with greater precision and efficiency.
One of the most important milestones in the history of CRISPR was the first demonstration of genome editing in human cells. In 2013, a team of scientists led by Feng Zhang of the Broad Institute in the US published a paper describing the technology and demonstrating that genetic modifications could be achieved in just a couple of weeks. This breakthrough opened up new possibilities for medical research and the development of gene therapies.
Another important milestone in CRISPR research was the application of the technology to correct genetic mutations in animal models. In 2014, a team of scientists led by Kathy Niakan at the Laboratory of Molecular Biology in the UK used CRISPR to correct a genetic mutation in mouse embryos to demonstrate that CRISPR could be used to treat inherited genetic diseases. A few years later, the same team used the technology in human embryos, with the permission of the UK health authorities. The possibility of modifying the genetic sequence of human embryos has generated a great deal of controversy in the international scientific community, due to the ethical and philosophical implications of being able to programme people "à la carte" far beyond simply deleting some hereditary problems.
Despite regulatory challenges and ethical dilemmas, CRISPR research has continued to advance at a dizzying pace. As we will see below, new applications of the technology have been developed, such as genome editing in plants, modification of gut microbiota and detection of infectious diseases. In addition, scientists are exploring the possibility of using CRISPR to develop gene therapies for a wide range of diseases beyond sickle cell disease, from cancer to rare genetic diseases.
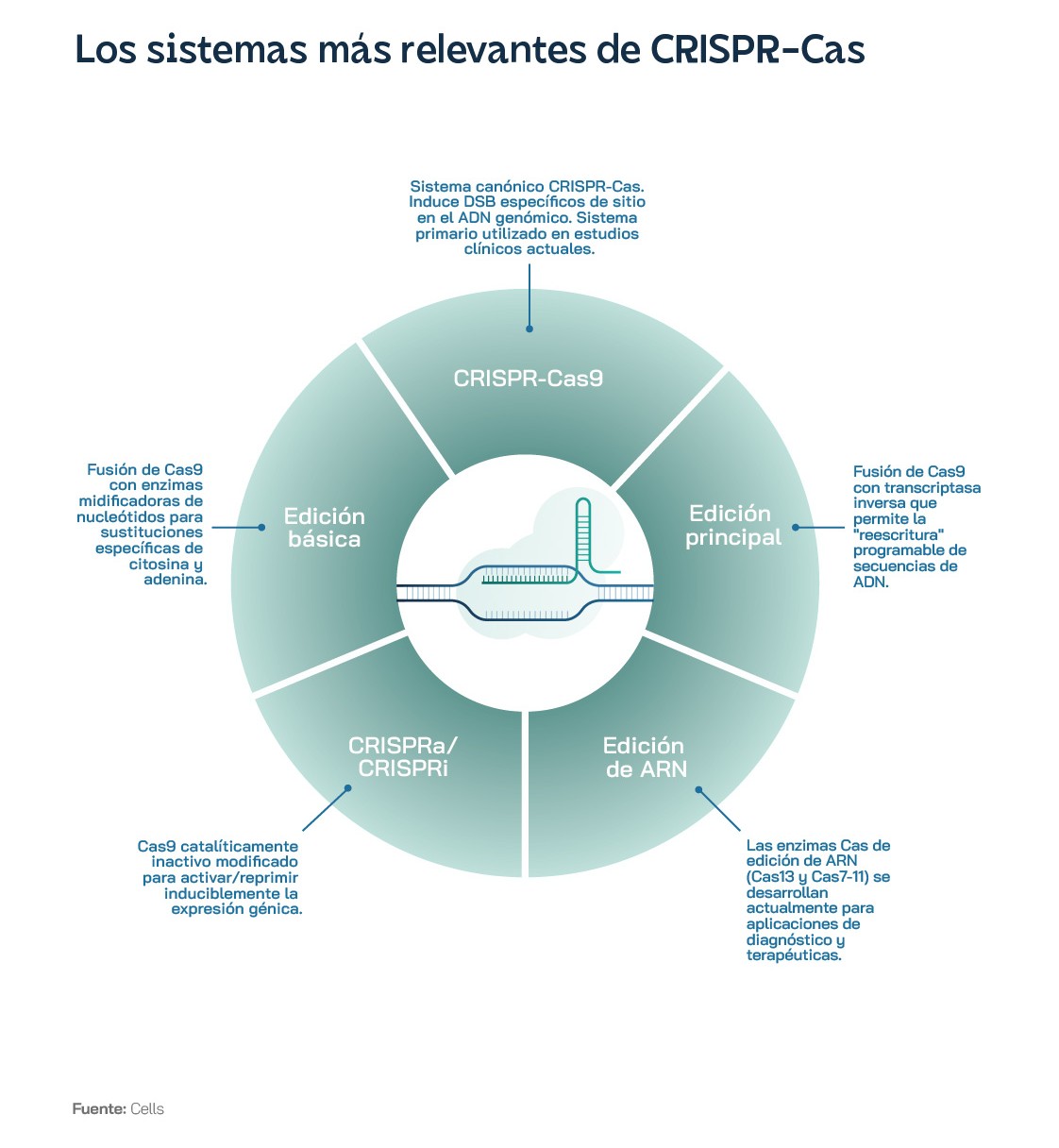
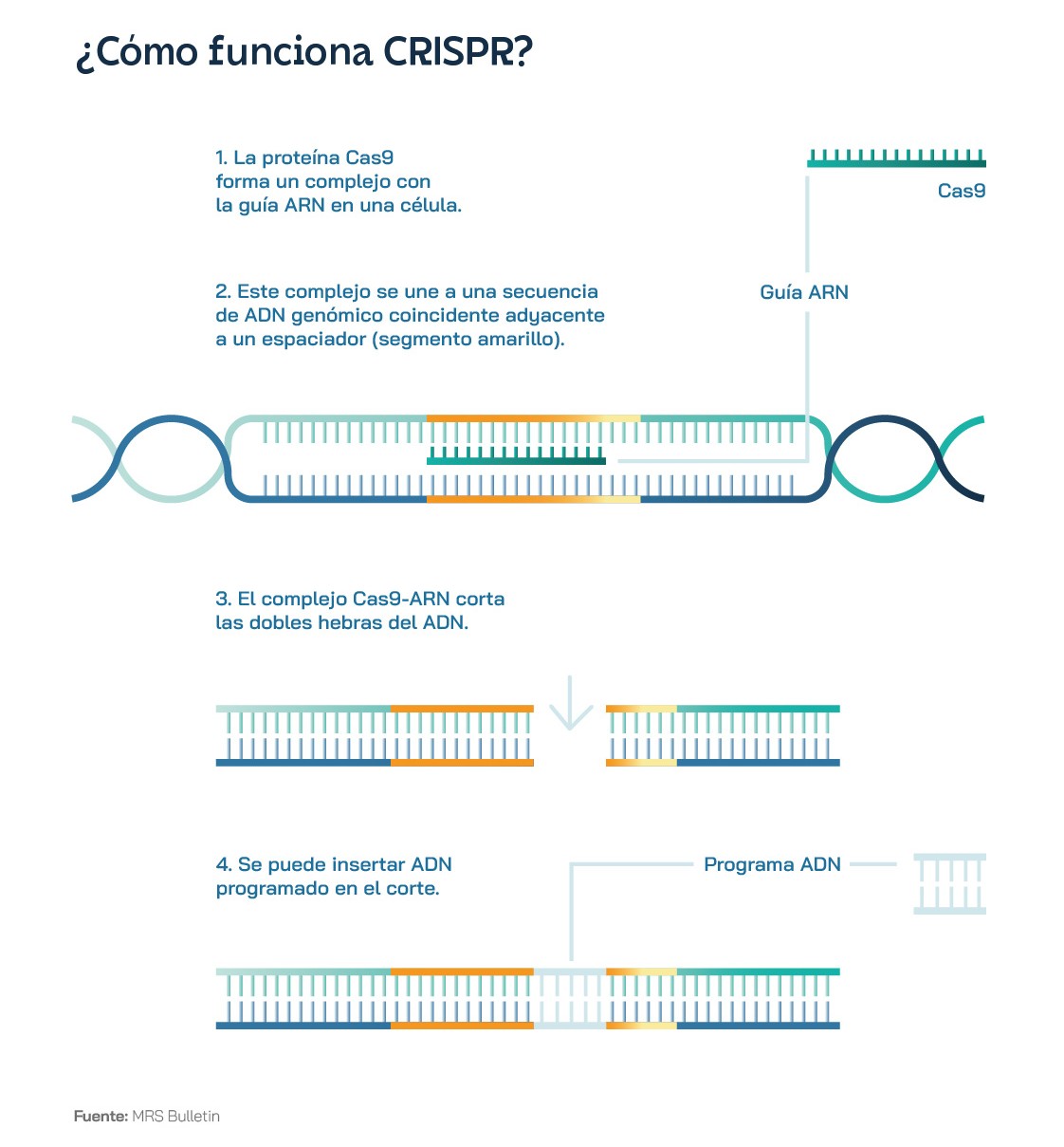
Clearly, CRISPR-Cas9 gene-editing technology has revolutionised the field of biotechnology and will soon revolutionise precision medicine, thanks to its ability to edit DNA accurately and efficiently. But how exactly does this powerful tool work? The key is a protein called Cas9, which acts like a molecular scissor, capable of cutting DNA fragments. Guided by a single strand of RNA, known as guide RNA, the Cas9 protein can precisely locate and cut DNA. Using this ingenious solution from the bacterial immune system, scientists have created a 'cut-and-paste' system capable of editing the DNA of human, animal and plant cells. Instead of targeting the genome of a virus, as would occur naturally in bacteria, guide RNA synthesised in the lab directs Cas9 to a specific DNA sequence in the genome.
CRISPR technology thus consists of two main components: the guide RNA and the Cas9 protein. The guide RNA is an RNA molecule - like those used in some of the COVID-19 vaccines - designed to be complementary to the specific DNA sequence to be edited. In this way, the guide RNA sequence and the DNA sequence to be edited will fit together like a key in a lock. An analogy that is probably more visual and apt is the two ribbons of a zip. The guide RNA binds to the Cas9 protein, forming a complex that scours and "scans" the genome for a DNA sequence that matches the complementary sequence of the guide. Once these two fragments meet, the Cas9 protein stops moving along the DNA strand and begins to function as an enzyme capable of cutting the genetic code. Experts sometimes refer to this enzyme as a 'molecular scissor' that cuts the DNA at the specific site defined by the guide RNA. Once the Cas9-guide RNA complex has bound to the target DNA, the Cas9 protein cuts both strands of DNA, creating a double-strand break in the DNA. It is then the cell's own natural repair mechanisms that come into play.
These systems include, among others, non-homologous end joining repair and homologous recombination repair. The former simply joins the broken ends of DNA. The latter is much more interesting, because the cell uses a homologous DNA molecule as a template to repair the break caused by Cas9. This allows scientists to insert or replace specific DNA sequences in the genome. One of the most impressive features of CRISPR-Cas9 is its precision and efficiency. Among other things, it can cut DNA at the target site with superior efficiency to 90%.
All living things have a genome. Bacteria, fungi, plants, animals and humans all use the same molecular tools to store the instructions for life. This means that the applications of CRISPR are virtually endless. The technology can be used to edit the genome of plants to make them more nutritious - or even more productive and resilient to the problems created by climate change - to stop the transmission of inherited genetic diseases, to create new tools in our fight against bacteria and especially resistant bacteria, and to edit the genome of human embryos. The latter has complex ethical implications, but could help to eradicate diseases such as haemophilia, cystic fibrosis and many others.
In agriculture, scientists are beginning to explore applications of CRISPR to edit the genes of different crops to make them tastier, more nutritious, even more resistant to weather and stress, two factors that will increase the risk as the climate crisis progresses. For example, CRISPR could be used to reduce the amount of allergens in peanuts or allow bananas to survive a deadly disease caused by pathogenic fungi. Recently, large multinational companies such as Monsanto and DuPont have begun buying licences to exploit CRISPR technology to develop new and more interesting crop varieties, to complement their research into traditional GM techniques. Compared to these techniques developed in the 1970s and 1980s, CRISPR offers a much faster, more accurate and versatile gene-editing alternative that can help identify genes associated with desirable traits more effectively.
CRISPR is also accelerating the detection and treatment of genetic diseases, including genome editing to eliminate hypertrophic cardiomyopathy, Huntington's disease, cystic fibrosis, and BRCA-1 and 2 mutations linked to breast and ovarian cancers. However, so far the only approved application of CRISPR technology is the treatment of sickle cell disease, a genetic and inherited condition that affects red blood cells, giving them a sickle shape instead of the normal, round shape, which causes a number of health problems. This is a special case, because it only requires the editing of one "letter" of DNA and can be applied directly in adults. So far, the technology has been seen to reduce problems and pain in more than 100 patients for at least a year, although the long-term effects are unknown. There are still hurdles to overcome before anyone begins other human clinical trials, for the treatment of more complex diseases. For example, Cas9 enzymes can occasionally "misfire" and edit DNA in unexpected places, which could lead to cancer or even create new diseases. Some experts believe that CRISPR's ability to wreak havoc and cause serious problems in DNA has been "severely underestimated". Scientific studies are now focused on identifying these problems, implementing the therapies slowly and cautiously, and developing new advances that will further improve the accuracy and long-term safety of CRISPR technology. Among other advances, new proteins and enzymes similar to Cas9, such as Cas12 and Cas13, are being developed to overcome some of these drawbacks and make gene editing systems more precise.
The fight against antibiotic-resistant bacteria and infectious diseases such as the Zika virus, whose incidence is growing due to the effects of the climate crisis, could also be tackled with CRISPR. In the first example, gene editing could bridge the development of new antibiotics by developing new targeting strategies that modify the DNA of pathogens. These techniques are also being used against viral infections such as AIDS and herpes infections. CRISPR could also be used in 'directed engineering' techniques to modify the genetic code not just of an organism, but of an entire species through propagation and inheritance. This would allow, for example, mosquitoes, vectors of diseases such as Zika and malaria, to be genetically modified to produce only male offspring. Eventually, the population would become extinct. Mosquitoes could also be made resistant to parasites, to prevent the transmission of diseases to other species, including humans.
More recently, promising results have been presented that use CRISPR to improve cancer therapies, thereby improving efficacy through genetic reprogramming for more personalised treatments. In an initial clinical study involving 16 patients with different types of cancer, including colon, breast and lung cancer, a group of scientists led by Spaniard Antoni Ribas used CRISPR to improve immunotherapy against these tumours. Thanks to gene editing, the scientists were able to reprogramme patients' immune systems to specifically attack cancer cells, while leaving healthy cells untouched. This breakthrough offers new hope in the fight against cancer. Our immune system naturally fights cancer cells every day. The problem is that, as cancer grows, the ability to fight the disease progressively diminishes. This technology, however, is able to isolate cancer cell-specific receptors and antibodies in blood samples and, after extensive analysis, uses CRISPR to programme the rest of the immune system's cells, which "learn" to create the same antibodies and kill the cancer more precisely and more effectively. This personalised approach, using the patient's own cells and receptors, reduces the risk of side effects and increases the likelihood of treatment success.
Despite the great potential of this technology to revolutionise agriculture, biotechnology and precision medicine treatments, CRISPR poses a number of ethical, social and technical challenges that need to be rigorously addressed. First, in terms of ethical considerations, the ability to edit the genome - especially the human genome - with technologies such as CRISPR-Cas9 and its improved versions raises a number of difficult questions. For example, debates arise over the use and modification of human embryos, given that in addition to correcting health-related problems, other factors such as intelligence or physical appearance could be altered, a concept that borders on the horrors of eugenics and artificial selection. Such genetic editing could alter the genetic diversity of the species - one of the main evolutionary advantages - and open the door to the "engineering" of human beings, a phenomenon that would only increase the differences and inequalities in society.
In addition to ethical considerations, there are technical challenges related to reproducibility and safety. For example, the system does not always cut DNA accurately and may cause unwanted mutations. Improving the accuracy and efficiency of the technology is essential to ensure its safety and effectiveness. In addition, concerns about CRISPR's ability to edit multiple gene sequences simultaneously and the possible unintended effects of these edits must also be addressed. Finally, many questions remain to be answered in the field of patents - one of the most publicised intellectual property cases in recent years - and the regulation of CRISPR-Cas9 technology. The technology poses regulatory challenges at different levels and requires strict and extremely clear legislation on how and when it can be used. There is a need to ensure that CRISPR is used ethically and safely, to avoid misuse or irresponsible use. Jennifer Doudna, one of the pioneers of CRISPR, is currently working to spread and debate the ethical and legal implications of gene-editing technologies and, among other things, has co-founded a research institute that promotes "the common good" to develop solutions against disease, hunger and social problems such as the climate crisis. In short, this revolutionary tool discovered on the Costa Blanca has the potential to transform medicine, agriculture and biotechnology, but it poses a number of ethical, social and technological challenges that must be rigorously addressed to minimise risks and protect the well-being of society.
Rebuilding health from the genes
The UK's Medicines and Healthcare products Regulatory Agency was the first authority to approve Casgevy in November 2023. It is the first CRISPR therapy for sickle cell disease and transfusion-dependent beta thalassaemia (TDT), developed by US-based CRISPR Therapeutics in partnership with Ireland's Vertex. The US Food and Drug Administration (FDA) followed in its footsteps, with initial approval for sickle cell disease in December 2023 and for TDT in January 2024. Finally, the European Commission endorsed the positive opinion adopted by the European Medicines Agency (EMA) in December 2023, but ultimately granted a conditional marketing authorisation for one year, renewable annually as more clinical data are reported.
Despite the first wave of cell and gene therapy approvals, the industry is still in its infancy and geographic differences between regulatory authorities do not help to clarify the playing field. The biotech sector associated with CRISPR has yet to adapt regulations to its novel needs, and states are opting for the most stringent positions until the remaining open questions are resolved. Quality standards need to be set to enable strategies to be put in place to help scale up the technology. In the meantime, there is the paradox that the first chimeric antigen receptor (CAR) T-cell therapy, Kymriah The cost of developing a new cell or gene therapy is around $1.94 billion, taking into account the research and development drop-out rate, but from the perspective of public policymakers we live in a world where a modestly funded lab with a small team of trained technicians can use CRISPR to alter the human genome, and that is both exhilarating and terrifying. Europe has warned, for example, that there is an urgent need to define and harmonise the science-based criteria that must determine the severity of a disease for genome editing to be justified. Uncontrolled activity can pose major social and ethical risks, so it is in favour of a strict regulatory framework. In this respect, the European Parliament is not satisfied with the term "human enhancement" as a criterion for this type of intervention, considering it to be vague, value-laden and difficult to apply. It prefers to establish the types of editing that should be banned or restricted, and to adopt a multi-level, risk-based approach.
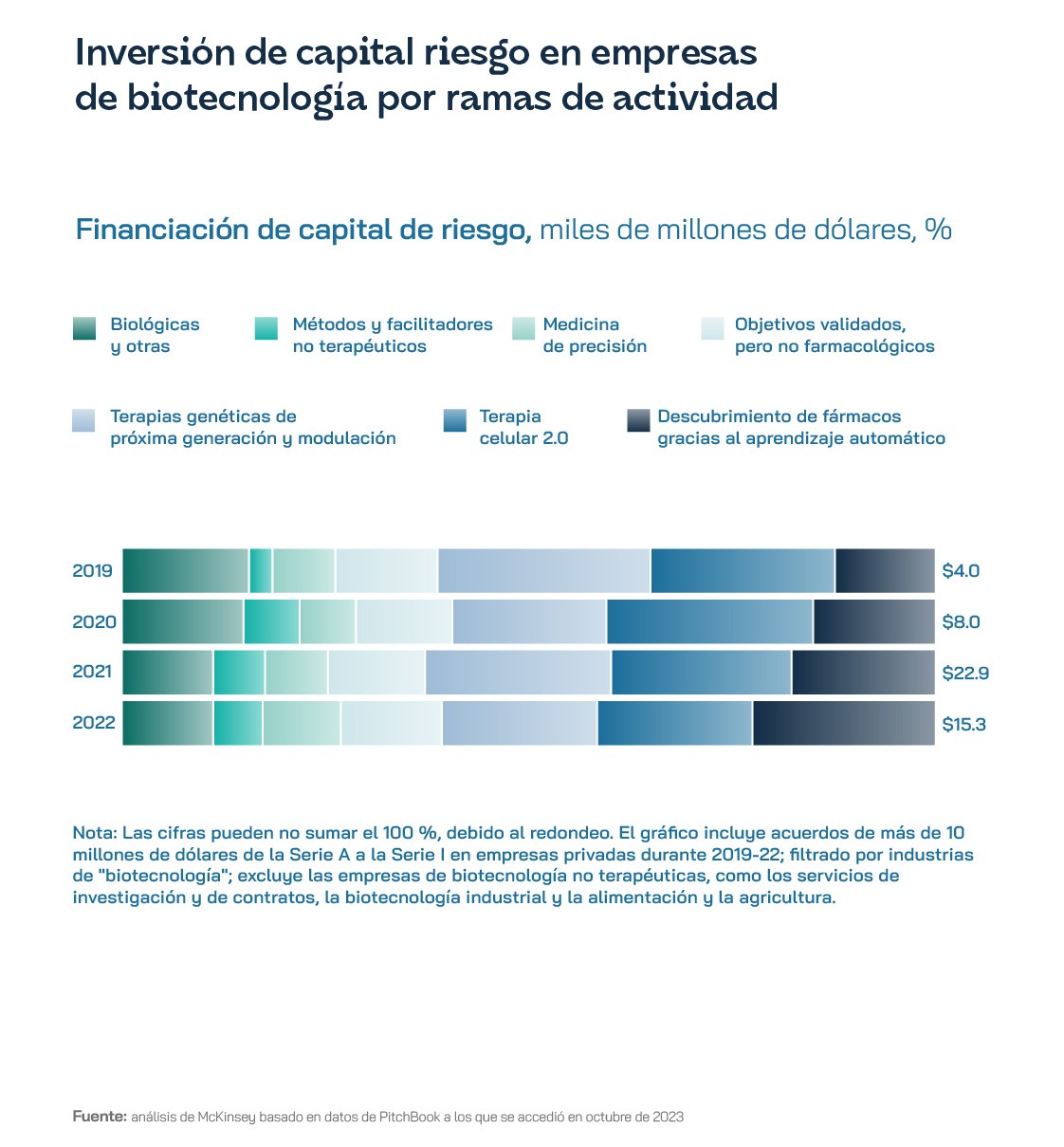
Given that the market is fragmented, contributions to the regulatory coordination effort must come not only from national governments, but also from supranational institutions. This ultimately translates into uncertainty for investors, an indeterminacy that is conditioning the development of an industry that has a first-rate scientific-technological substratum in Western countries. European-based investment funds have grown significantly since 2010 and are achieving net internal rates of return on late-stage investments of 15%, compared to 13% in the US. However, the US has 22 biotech funds of more than $1bn, compared to only one of this size in Europe. Regulatory hesitancy also does not make it easy to foster a risk-taking entrepreneurial culture: the European market accounted for only 24% of the world's biotech start-ups between 2018 and 2020, compared to 65% in the US. China has gone from 8% to 12% in that time.
The venture capital and start-up data stand in stark contrast to the fact that the main vaccines against COVID-19 were developed by scientists in Europe: Pfizer-BioNTech and AstraZeneca. Even more so in the case of Spain, where researcher Francis Mojica discovered the theoretical principles of CRISPR, but not a single patent related to his work has been registered. It should come as no surprise that 95% of European biotech companies listed in the US are listed on the Nasdaq. The market for cell and gene therapies developed from both allogeneic (the cell material comes from different individuals of the same species) and autologous (it is from the same individual receiving the treatment) sources is expected to reach almost $160 billion by 2028. Autologous medicines will lead the way, contributing $83 billion, compared to $74 billion for allogeneic therapies. Most of the 3,951 cell and gene therapies in development were in the preclinical phase at the beginning of 2024, with the next largest group being discovery-stage therapies. A quarter of these were designed to treat different forms of cancer, followed by diseases affecting the central nervous system and ophthalmic conditions. Gene therapies, including genetically modified cell therapies such as CAR T-cell therapies, accounted for 2,111 cases, while genetically modified cell therapies amounted to 878. Of the 97% CAR-T cell therapies in development, 97% were aimed at suppressing different cancer variants and the remainder included scleroderma, HIV/AIDS and autoimmune diseases. Of the 1,038 gene therapies in development for rare diseases, eight of the top 10 were also oncology, led by myeloma, acute myelogenous leukaemia, non-Hodgkin's lymphoma, B-cell lymphoma and ovarian cancer.
With citizens in mind, the key is that all this unfinished business of strategic harmonisation does not hinder access to experimental or newly approved therapeutic options, including participation in clinical trials. In this respect, it is logical to recognise that the traditional clinical trial pathway for a treatment that might end up being administered to 10 patients per year, as is the case for gene therapies, cannot be the same as for a treatment that will be administered to 100,000 or millions, as is the case for the most popular drugs. To facilitate minority treatments, Europe has instituted the Hospital Exemption Rule, designed to benefit people with rare diseases. In Spain, the authorisation of these non-industrially manufactured advanced therapy drugs is regulated by a Royal Decree, according to which the drugs are authorised for use in a hospital setting and only for the treatment of an individual patient.
The problem is that the spectrum of technologies keeps getting more and more complicated: from the initial CRISPR Cas9, which was set up as a simple editing system, we have moved on to a wide range of editors, base editors and prime editingThe latter are much more complex. Alongside this, customised products need to be reconciled with the development of repeatable processes that yield reproducible results, which is increasingly critical in the eyes of regulators. One key is to make it easier for innovators to work with authorities as early as possible on product lifecycle analysis to understand their approval requirements. The US also aims to improve harmonisation with other regulators in the field of gene therapies, especially in the case of rare diseases, which affect few individuals. In addition, its strategy includes supporting small companies and, to this end, the FDA has launched a pilot Supporting Trials Advancing Rare Disease Therapy (START) programme, which uses methods similar to those once used by the Warp Speed Project, during the development of the COVID-19 vaccine, to support the development of gene therapies.
The expected increase in demand also raises questions about potential limitations in current and future manufacturing capacity for CRISPR-based medical solutions. Manufacturing cell therapy drugs at scale would involve producing batches of up to 2,000 litres in single-use disposable formats, limiting the options for any one biotech company. If the therapy could reach hundreds or thousands of patients, companies would have to generate between 1011 y 1014 cells per year, which would place a heavy burden on the operations area. Processes that enable faster delivery of CAR T therapy will therefore be of critical value once this treatment option becomes the standard of care. In the area of vaccines, there is concern about the well-documented shortage of viral vectors, given that the number of approvals is expected to grow over the next five years and there are relatively few contract manufacturing sites worldwide. The same need for increased efficiency applies to the production of lentiviral vectors for genetically modified cell therapies, where rapid and optimised downstream processing is needed due to stability issues over time.
The question of price is opening up a fierce ethical debate. While single doses of CAR T therapy cost a few hundred thousand dollars, commercially available gene therapies are worth millions. Vertex and CRISPR Therapeutics set a price of $2.2 million for Casgevy's single treatment, although as of early 2024 they had not finalised what price they would charge in Europe, where access is determined by negotiations with national authorities. Vertex has organised early access for DTT patients in France prior to national reimbursement and planned to open 25 authorised treatment centres in Europe, three of which were operational at the time of the announcement. On the same day that the FDA approved Casgevy for sickle cell disease, it also gave the go-ahead to Bluebird Bio's Lyfgenia cell-based gene therapy, adding to the August 2022 approval of its Zynteglo gene therapy for treating DTD. Lyfgenia had a price tag of $3.1 million in 2024, while Zynteglo's was $2.8 million. On top of the access and pricing issues, there are risk considerations. The chemotherapy required before CRISPR treatment is hard on patients and carries the risk of serious side effects.
There are several areas that manufacturers can focus on to reduce the cost of therapies. Traditional manufacturing methods involve manual processes performed by highly skilled and experienced workers in a cleanroom environment. Transitioning manufacturing to an automated environment is seen as a critical step to increase efficiency. In the field of cell therapy, a complementary innovative approach to reducing costs is emerging, with companies such as Umoja Biopharma leading the way, involving the use of gene therapy to allow the body itself to manufacture engineered cells for treatment, bypassing the traditional manufacturing bottleneck and opening up new possibilities for more effective and personalised solutions.
Another determining factor for the viability of new therapies is the high price of raw materials, in particular specialised cell culture media rich in growth factors and cytokines. The development of a more efficient generation of cell culture media, with greater use of recombinant components, could help to make therapies cheaper. Finally, innovations aimed at creating 'off-the-shelf' products remain a critical focus in this field, and companies such as Be Biopharma and Neurona Therapeutics are actively working on allogeneic treatments that have the potential to reduce costs.
These constraints were compounded in 2023 by a striking loss of investor interest following the outbreak associated with the COVID-19 pandemic. Venture capitalists want to see profitability on the horizon and this is pushing biotech companies to focus on clinical trials in order to bring new products to market as quickly as possible. Because of the high price tag, several CRISPR-focused companies have cut staff, in an environment of widespread layoffs involving more than 250 firms in the sector between 2022 and 2023. They have decided to focus on their most developed products, rather than tackling new treatments and expanding into other disease areas. The S&P Biotechnology Select Industry Index closed the fourth quarter of 2023 one 50% below its February 2021 peak. That year only 30 biotech companies had completed an IPO (Initial Public Offering) in the first three quarters of 2023 compared to 114 in 2021, and capital raised amounted to just $3.4bn, down from $16bn two years earlier. An indicator of a greater propensity for conservatism may be that, in the fourth quarter of that year, the number of gene therapies in Phase III clinical trials grew by 10%, the first such quarterly increase since the third quarter of 2022.
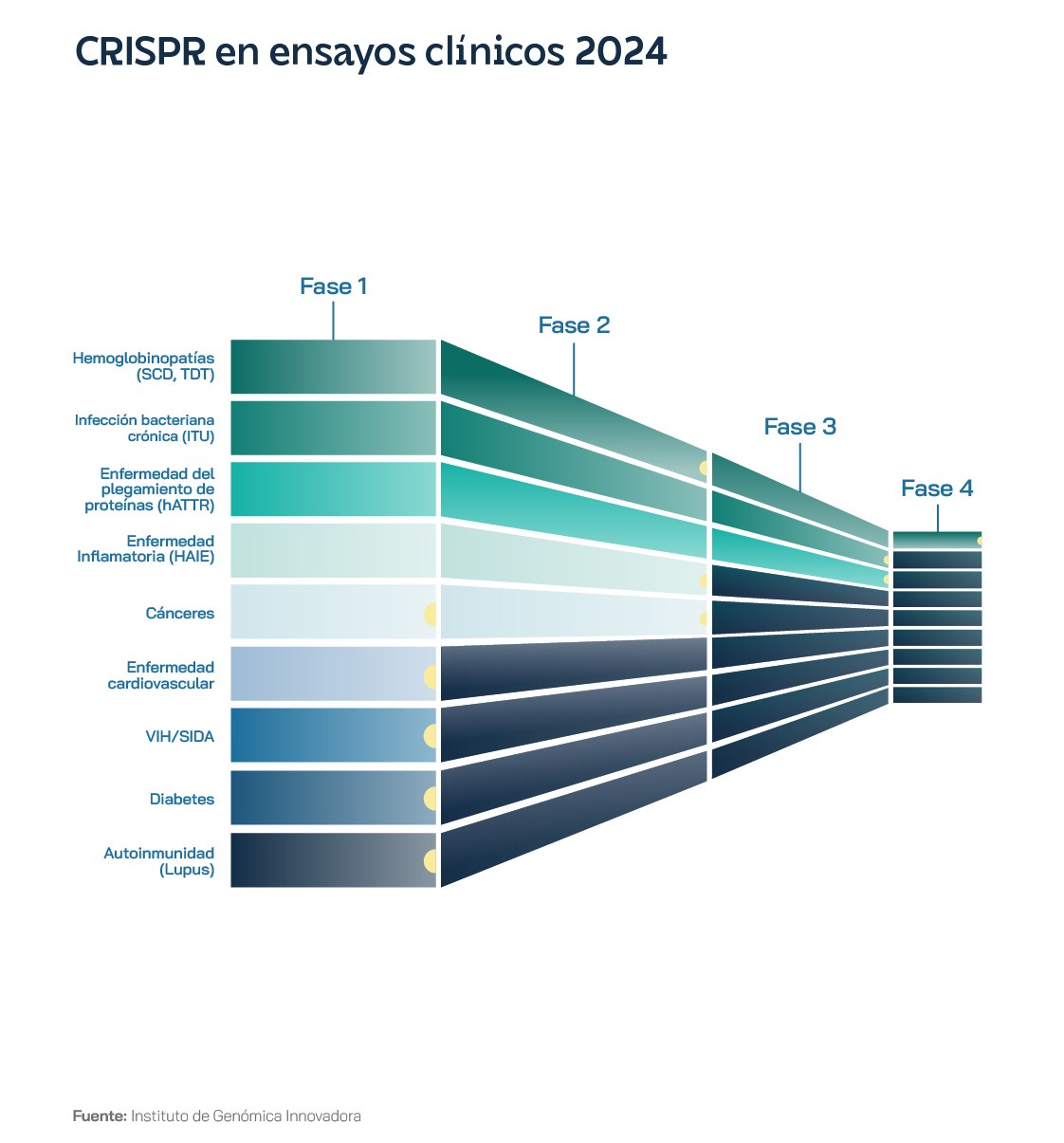
At the beginning of 2024, only one trial in a single new disease area was planned for the whole year: autoimmunity. Editas Medicine was conducting phase 1/2 evaluations for people with severe SCD and TDT, using a CRISPR system with the Cas12a protein, rather than the more famous Cas9, in what was the first case of Cas12 being used in a clinical trial. Also in January 2024, Beam Therapeutics announced that it had dosed the first participant in its phase 1/2 trial in the US of a base editing therapy for severe SCD. Base editing is a version of CRISPR-Cas9 technology that changes a single letter of DNA, without creating breaks in the DNA double strand, which reduces certain risks, and so has attracted interest from investors. Until now, all therapies were ex vivo: cells were removed from the body, edited and quality-controlled in a special laboratory, until they were returned to the body after the patient underwent intensive chemotherapy. In vivo therapy would mean administering the gene-editing drug directly into the person's body, where the cells are edited.
Despite the tough backdrop, companies such as Scribe Therapeutics, in CRISPR engineering by design, Sail Biomedicines, in RNA programming, and ADARx Pharmaceuticals, in RNA editing, underlined the industry's willingness to commit to novel technologies even as existing approaches such as CRISPR-Cas9, CAR-T and TCR are still in development. A notable trend is the growing collaboration with new contract manufacturing and development start-ups, such as ElevateBio and Resilience, to leverage their expertise in specialised manufacturing. CRISPR fusion proteins and new nucleases are also being investigated for their ability to facilitate targeted integrations in large gene sequences so that, rather than repairing individual mutations, larger defective genomic sequences can be completely replaced with corrected versions, potentially expanding the impact of gene editing to more diseases.
The US FDA's strategy is to speed up the approval of platform technologies. The Omnibus Appropriations Act of 2023 contains provisions to facilitate the approval of new gene therapies. A platform technology applies when all parts of a treatment are standardised in a single commercially available package and only certain parts change for a given disease. For CRISPR-based treatments, the standardised part would include the Cas protein, the method of delivery to the cells, the administration of the treatment and the dose the patient receives. The only thing that would change would be the sequence of the guide RNA and the DNA repair template to be specified for different edits. By early 2024, CRISPR Therapeutics said it was well positioned to execute clinical trials in areas such as oncology, autoimmunity, cardiovascular and diabetes, with the prospect of results over the next 12 to 18 months, open to innovate on its platform of next-generation gene editing and delivery technologies, even in the challenging macroeconomic environment for biotech companies.
Ultimately, CRISPR technology will always be associated with the patent battle. Since the United States Patent and Trademark Office (USPTO) granted US Patent No. 8,697,359 to the Broad Institute, MIT and scientist Feng Zhang in April 2014, the CRISPR components and methods contained therein have become the leading standard for genome editing worldwide. The patent owners have openly shared CRISPR reagents and tools with more than 3,000 institutions in 75 countries through the non-profit organisation Addgene. For corporate research, the Broad Institute licenses CRISPR IP through an open "inclusive innovation" model that maximises opportunities for therapeutic development in human disease. The patents issued by Broad are for genome editing and its use in eukaryotic cells, including animal, human and plant cells. The institute insists on differentiating them accordingly from those issued by the University of California-Berkeley (UCB), which are not specific to uses in eukaryotic cells, and on asking UCB to join discussions on a patent pool or other coordinated licensing approach, such as the one it developed for CRISPR in agriculture. In Europe, the Broad Institute has been granted 33 CRISPR patents, including 29 patents related to CRISPR-Cas9 and four related to CRISPR-Cas12/Cpf1, but both these and those approved for UCB have been opposed by multiple parties, usually on technical issues in the application.
Basic science to be transferred
International events such as The CRISPR MEDiCINE Conference in Copenhagen (Denmark) highlight the potential of Spanish basic science in the development of techniques associated with health. It is well above the level of development achieved by industry so far to scale up these solutions.
Gene editing has been used for the acute correction of a wide range of disease-associated mutations. But in certain cases, such as Fanconi anaemia (FA), the more conventional homology-directed DNA repair mechanism does not work and alternative gene therapy approaches are required. CIEMAT/CIBERER in Madrid has optimised the use of base editing and principal editing to potentially correct most of the mutations described in patients, with the ultimate goal of generating more personalised drugs. In addition, together with the Fundación Instituto de Investigación Sanitaria de la Fundación Jiménez Díaz, Universidad Carlos III, IMDEA Nanociencia and Hospital Ramón y Cajal, they are also investigating mechanisms to prevent the effects of debilitating palmoplantar keratoderma, associated with distressing blisters that severely limit their ability to walk, that patients with pachyonychia congenita, a rare inherited skin disorder, often develop. CRISPR tools open up possibilities for gene therapy protocols to address this condition.
Moreover, the use of paired Cas9 nickases for gene disruption in vivo has been shown to provide a potential pathway to decrease adeno-associated virus insertion frequencies. However, this technique requires further research to understand the factors influencing the choice of staggered double-strand break repair pathways. Researchers at the University of Navarra and Harvard Medical School are currently pursuing this line of research.
Spanish centres at the University of Barcelona, CIBERER, Instituto de Salud Carlos III (ISCIII), IBUB and IRSJD, together with Lund University (Sweden), are working on neurodevelopmental disorders caused by different disturbances during early brain development. Many of them have a genetic origin, but most of the underlying genetic alterations remain unknown. The challenge was to advance our understanding of this process given the limited access to human brain tissues in early development and the difficulty of using animal models. The project has used state-of-the-art in vitro models coupled with next-generation gene sequencing and editing techniques to study the effects of a range of mutations. Among other actions, CRISPR-edited induced pluripotent stem cell lines incorporating two of the mutations studied were generated, leading to critically important findings. The same Spanish players have worked on another promising approach to pain relief based on the inactivation of two enzymes that modulate endocannabinoid levels, laying the groundwork for exploring the therapeutic potential of gene editing for this purpose in the peripheral nervous system.
The international connection of our research ecosystem has also allowed CIEMAT and UC3M, in addition to the "12 de Octubre" University Hospital in Madrid, to work with University College Dublin and Anhui University of Science and Technology (Huainan, China) on a lung cancer platform that uses synthetic biomaterials for the delivery of CRISPR/Cas9 reagents to adult lung epithelial cells. It has proven to be an efficient approach for modelling lung tumourigenesis by simultaneously inactivating a set of tumour suppressor genes.
In the field of transferring these advances to the entrepreneurial world, biotech companies such as Integra Therapeutics, which is creating next-generation genetic writing tools, stand out. It is based on FiCAT technology, derived from research in Dr Marc Güell's lab at Pompeu Fabra University and aimed at solving the current limitations in genetic writing: size, accuracy and stability. In mid-2024, a €2.5 million grant and an equity investment of up to €8 million from the European Commission was announced for the company through the European Innovation Council (EIC) Accelerator programme. Integra Therapeutics will use the initial €2.5 million for pre-commercialisation activities of its FiCAT platform for T-cell and haematopoietic stem cell (HSC) engineering to develop cell therapies for rare diseases, autoimmune diseases and oncology.